Sucrose: A Comprehensive Guide to Composition, Uses, and Health Implication , history
A Complete Guide to Sucrose: Everything You Need to Know
Sucrose, also known as table sugar, is one of the most common and widely used sweeteners in the world. But what is sucrose exactly, and how does it affect your health? In this article, we will explore the chemistry, production, uses, and health implications of sucrose, as well as some alternatives and interesting facts.
Introduction to Sucrose
Definition of Sucrose
Sucrose is a type of carbohydrate and a simple sugar, specifically a disaccharide composed of glucose and fructose. Sucrose has the scientific name of 1-D-fructofuranosyl–D-glucopyranoside. Sucrose has a molecular formula of C12H22O11 and a molecular weight of 342.3 g/mol.
Composition and Structure of Sucrose
Sucrose is made up of two monosaccharides, glucose and fructose, that are linked by a glycosidic bond between their anomeric carbons. This means that sucrose is a non-reducing sugar, meaning that it does not react with Benedict’s reagent or other oxidizing agents. The structure of sucrose can be represented as follows:
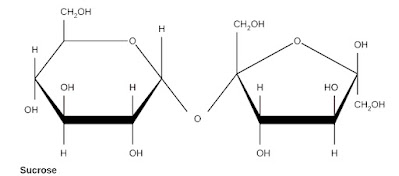 |
Image Attribution:( changes has been made by cropping out lactose and maltose) Author: Madprime License: CC BY 4.0 Source: Wikimedia Commons |
The bond between glucose and fructose can be broken by hydrolysis, which is catalyzed by the enzyme sucrase, also known as invertase. This results in the formation of equal amounts of glucose and fructose, which are called invert sugar.
Sources of Sucrose in Nature
Sucrose is a natural plant sugar that is produced by photosynthesis. It is stored in various parts of plants, such as stems, roots, fruits, flowers, and seeds. It is mainly found in nature in sugar cane and sugar beet, which have been used as rich sources of sucrose for commercial purposes since the 17th century.
Other sources of sucrose include maple syrup, honey, agave nectar, coconut sugar, date sugar, and molasses.
Properties and Characteristics of Sucrose
Sweetness and Flavor Profile
Sucrose is one of the sweetest natural sugars, with a relative sweetness of 1.0 compared with other sugars. However, it is less sweet than fructose alone but sweeter than glucose alone. The concentration, temperature, pH, and other substances in the solution affect how sweet sucrose tastes. Sucrose has a pleasant flavor that enhances the taste of many foods and beverages.
Solubility in Water
Sucrose is highly soluble in water, with a solubility of 211 g/100 mL at 20°C. The solubility of sucrose changes ( increase) with (increase) temperature and pressure. It also depends on how pure the sucrose is and what other solutes are in the solution.
Crystalline Structure
Sucrose can form crystals when it is dissolved in water and then evaporated or cooled down. The crystals have a monoclinic shape and are transparent or white in color. The size and shape of the crystals depend on the conditions of crystallization, such as temperature, concentration, agitation, impurities, and seeding. The crystals can be further refined by washing, drying, sieving, or milling.
Stability and Shelf Life
Sucrose is relatively stable under normal conditions, but it can undergo various chemical reactions under certain circumstances. Some of these reactions include:
- Hydrolysis: Sucrose can be hydrolyzed by water or acids to form glucose and fructose. This reaction is accelerated by heat and enzymes.
- Caramelization: Sucrose can be heated above its melting point (185°C) to form caramel, which is a brown-colored mixture of various compounds, such as furans, lactones, and caramelans. This reaction gives a characteristic flavor and color to many foods, such as candies, cakes, and sauces.
- Maillard reaction: Sucrose can react with amino acids or proteins to form various compounds, such as melanoidins, which are brown-colored and have complex flavors and aromas. Sucrose undergoes a reaction that causes browning and flavor changes in many foods, such as bread, coffee, and meat.
- This reaction happens at low moisture and high temperature conditions.
The shelf life of sucrose depends on the storage conditions, such as temperature, humidity, light, and air. Sucrose can last for several years if stored in a cool, dry, dark, and airtight container. However, it can degrade over time due to moisture absorption, microbial growth, insect infestation, or chemical reactions.
Production and Extraction of Sucrose
Sucrose in Plants
Sucrose is synthesized in the leaves of plants by the process of photosynthesis, which converts carbon dioxide and water into glucose and oxygen. The glucose is then converted into fructose by the enzyme fructokinase. The glucose and fructose are then joined together by the enzyme sucrose-phosphate synthase to form sucrose-phosphate, which is then dephosphorylated by the enzyme sucrose-phosphate phosphatase to form sucrose. The sucrose is then transported to other parts of the plant by the phloem, which is a system of tubes that carry sugars and other nutrients throughout the plant. The sucrose is stored in various organs of the plant, such as stems, roots, fruits, flowers, and seeds.
Harvesting and Processing of Sucrose
The most common sources of sucrose for commercial production are sugar cane and sugar beet. Sugar cane is a tropical grass that grows up to 6 meters tall and has thick stems that contain 10–20% sucrose by weight. Sugar beet is a root crop that grows in temperate regions and has roots that contain 15–20% sucrose by weight. The harvesting and processing of sucrose from these plants involve the following steps:
- Harvesting: The sugar cane or sugar beet is harvested when it reaches maturity, usually after 6–18 months of growth. The sugar cane is cut by hand or machine and transported to the mill. The sugar beet is lifted by machine and transported to the factory.
- Crushing or slicing: The sugar cane or sugar beet is crushed or sliced to extract the juice that contains sucrose and other impurities.
- Clarification: The juice is clarified by adding lime and heating it to remove impurities, such as dirt, fibers, proteins, and minerals. The impurities form a solid mass called bagasse in sugar cane or pulp in sugar beet, which can be used as animal feed or fuel.
- Evaporation: The clarified juice is evaporated by boiling it under vacuum to concentrate the sucrose content. The resulting syrup is called thick juice in sugar cane or thin juice in sugar beet.
- Crystallization: The thick or thin juice is further evaporated and cooled to form crystals of sucrose. The sucrose crystals are separated from the leftover liquid called molasses by spinning (centrifugation) them rapidly. The crystals are then rinsed with water to get rid of any molasses left on them.
- Drying: The crystals are dried by hot air or steam to reduce the moisture content. The dried crystals are called raw sugar in sugar cane or white sugar in sugar beet.
Refining and Purification Methods
The raw or white sugar can be further refined and purified to produce different grades of sucrose for various purposes. Some of the refining and purification methods include:
- Affination: The raw sugar is mixed with a concentrated syrup called affination syrup to dissolve the outer layer of molasses. The mixture is then centrifuged to separate the crystals from the syrup. The crystals are then washed with water to remove any remaining syrup.
- Carbonatation: The affinated sugar is dissolved in water and treated with carbon dioxide gas and lime to form calcium carbonate precipitates that trap impurities. The mixture is then filtered to remove the precipitates.
- Decolorization: The carbonated sugar solution is treated with activated charcoal or bone char to remove any colorants. The solution is then filtered again to remove the charcoal or bone char.
- Crystallization: The decolorized sugar solution is evaporated and cooled again to form crystals. The crystals are then sieved or milled to produce different sizes and shapes of sucrose, such as granulated, powdered, or brown sugar.
Uses and Applications of Sucrose
Food and Beverage Industry
Sucrose is widely used in the food and beverage industry as a sweetener, flavor enhancer, texturizer, and stabilizer. Some of the common uses of sucrose in this industry are:
- Sweetening agent: Sucrose is added to many foods and drinks to improve their taste and palatability. Examples include candies, chocolates, cakes, cookies, ice cream, jams, preserves, sauces, syrups, soft drinks, energy drinks, coffee, tea, and fruit juices.
- Flavor enhancer: Sucrose can enhance the flavor of many foods and drinks by balancing acidity, bitterness, saltiness, or spiciness. Examples include tomato ketchup, barbeque sauce, salad dressing, pickles, yogurt, cheese, bread, wine, and beer.
- Texturizer and stabilizer: Sucrose can modify the texture and consistency of many foods and drinks by influencing their viscosity, crystallization, gelation, freezing point, or moisture retention. Examples include caramel, fudge, marshmallow, nougat, honeycomb, meringue, whipped cream, ice cream, sorbet, sherbet, and frozen yogurt.
Pharmaceutical and Medical Applications
Sucrose is also used in the pharmaceutical and medical applications for various purposes. Some of them are:
- Excipient: Sucrose is used as an inactive ingredient or filler in many drugs and supplements to improve their taste, appearance, stability, solubility, or bioavailability. Examples include tablets, capsules, lozenges, syrups, suspensions, powders, granules, and chewables.
- Preservative: Sucrose is used as a preservative in some drugs and biological products to prevent microbial growth or degradation. Examples include vaccines, antibodies, enzymes, hormones, and blood products.
- Osmotic agent: Sucrose is used as an osmotic agent in some medical procedures to create a hypertonic solution that draws water out of cells or tissues. Examples include oral rehydration therapy for dehydration or diarrhea; intravenous infusion for hyponatremia or cerebral edema; peritoneal dialysis for kidney failure; and sclerotherapy for varicose veins.
- Demulcent: Sucrose is used as a demulcent in some cough syrups or lozenges to soothe irritated mucous membranes in the throat or mouth.
- ( Visit professionals for medical, health, research, practical or professional purpose)
Industrial Uses
Besides food and medicine, sucrose has some industrial uses as well. Some of them are:
- Biofuel: Sucrose can be fermented by yeast or bacteria to produce ethanol and carbon dioxide. Ethanol can be used as a renewable fuel for vehicles or power generation.
- Bioplastic: Sucrose can be polymerized by enzymes or chemical catalysts to produce polylactic acid (PLA), which is a biodegradable plastic that can be used for packaging or textile applications.
- Biosurfactant: Sucrose can be converted by microorganisms into biosurfactants such as rhamnolipids or sophorolipids. These are natural detergents that can be used for cleaning or emulsifying purposes.
Health Implications of Sucrose
Sucrose is a source of energy and calories for the body. However, excessive consumption of sucrose can have negative effects on health. Some of them are:
- Metabolism: Sucrose is digested by the enzyme sucrase in the small intestine into glucose and fructose. Glucose is absorbed into the bloodstream and used by the cells for energy or stored as glycogen in the liver or muscles. Fructose is absorbed into the liver and converted into glucose or fat. Excess glucose or fat can lead to insulin resistance, metabolic syndrome, or fatty liver disease.
- Blood sugar levels: Sucrose can cause rapid spikes and drops in blood sugar levels due to its simple chemical composition. This can affect mood, energy, appetite, and cravings. It can also increase the risk of diabetes and obesity by impairing insulin secretion and sensitivity.
- Dental health: Sucrose can promote dental caries or cavities by providing food for bacteria that produce acids that erode the enamel of the teeth. It can also cause plaque formation and gum inflammation.
- Cardiovascular health: Sucrose can increase the levels of triglycerides and low-density lipoproteins (LDL), also known as the “bad” cholesterol, in the blood. This can increase the risk of atherosclerosis or plaque buildup in the arteries, which can lead to heart attack or stroke.
- Inflammation: Sucrose can trigger inflammation in the body by activating the immune system and increasing the production of cytokines, which are chemical messengers that regulate inflammation. Chronic inflammation can contribute to various diseases, such as arthritis, asthma, cancer, and Alzheimer’s disease.
Sucrose Alternatives and Substitutes
Due to the health concerns associated with sucrose, many people look for alternatives or substitutes that can provide sweetness without the calories or negative effects. Some of the common sucrose alternatives and substitutes are:
- Natural and artificial sweeteners: These are substances that taste sweet but have little or no calories or sugar. They can be used to sweeten foods and drinks without affecting blood sugar levels or dental health. However, they may have other side effects or health risks depending on their type and amount. Some examples of natural sweeteners are stevia , monk fruit , and erythritol . Some examples of artificial sweeteners are aspartame , sucralose , and saccharin .
- Healthier options for sugar intake: These are ways to reduce or moderate the consumption of sucrose and other added sugars in the diet. They include choosing foods that are naturally sweet, such as fruits, vegetables, dairy products, and grains; limiting processed foods that contain added sugars, such as candies, cakes, cookies, ice cream, jams, preserves, sauces, syrups, soft drinks, energy drinks, coffee, tea, and fruit juices; reading nutrition labels and ingredient lists to identify sources of added sugars; using smaller amounts or less frequent servings of sucrose or other sweeteners; and using spices or herbs to add flavor instead of sugar.
Interesting Facts and Trivia about Sucrose
Sucrose is more than just a sweetener. It also has some interesting facts and trivia that you may not know. Here are some of them:
- Historical significance: Sucrose has played an important role in history, culture, and economy. It was first cultivated in Papua New Guinea 10 000 years ago, and then spread to other parts of the world through trade and colonization. It was considered a luxury item in medieval Europe, and a major commodity in the Atlantic slave trade. It also sparked wars, revolutions, and social movements.
- Sucrose in culinary traditions: Sucrose is used in various culinary traditions around the world to create delicious dishes and desserts. Some examples are caramel custard from France, baklava from Turkey, gulab jamun from India, tiramisu from Italy, and cotton candy from Iran.
Conclusion
Sucrose is a common and widely used sugar that has many properties, uses, and applications. However, it also has some health implications that require caution and moderation. There are also alternatives and substitutes that can provide sweetness without the calories or negative effects of sucrose. Sucrose is more than just a sweetener. It is also a fascinating substance that has many interesting facts and trivia.
References
- Sucrose Facts and Health Effects | Nutrition
- Sucrose vs Glucose vs Fructose: What’s the Difference? - Healthline
- The Side Effects of Sucrose | Healthfully
- Both Sucrose and High Fructose Corn Syrup Linked to Increased Health Risks | UC Davis Health
- Artificial sweeteners and other sugar substitutes - Mayo Clinic
- Facts About Sugar and Sugar Substitutes | Johns Hopkins Medicine
- Common Uses of Sucrose | Healthfully
- Sucrose Alternatives and Similar Sites / Apps | AlternativeTo
- Structure, Properties, Uses, and FAQs of Sucrose. - BYJU'S
Comments
Post a Comment